Robert L. Chevalier, MD

Our research addresses the pathogenesis of congenital obstructive nephropathy, a group of malformations of the urinary tract representing the leading cause of kidney failure in children.
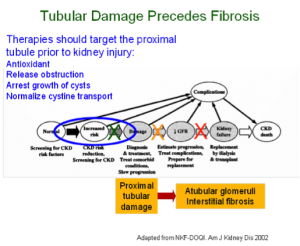
23 million Americans have chronic kidney disease (CKD), with a cost to the federal government exceeding $40 billion annually. Progression of CKD is inexorable regardless of its cause (most pediatric CKD is due to malformations of the kidneys and urinary tract, whereas most adult CKD is due to diabetes or hypertension). The “end-stage” kidney is characterized by generalized damage and scarring with loss of thousands of the kidney’s subunits (nephrons).
The animal model most widely used to study progressive CKD is the mouse subjected to unilateral ureteral obstruction (UUO). Our research team has developed novel microsurgical and microscopic techniques to show that obstructive nephropathy resulting from surgical UUO in the mouse causes previously unrecognized injury to nephrons, localized to the proximal tubule, which is highly susceptible to oxidative stress. Because recent clinical studies have shown that most congenital kidney disease progresses to end-stage renal disease in adulthood, our techniques have been adapted to the study of the lifespan of the animal, from birth to adulthood.
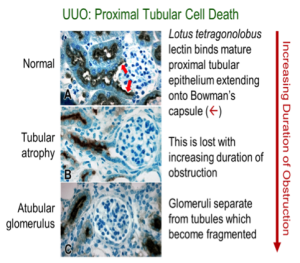
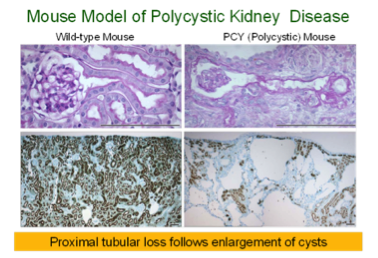
Most patients with severe congenital obstructive nephropathy have reduced nephron number, which accelerates progression. To examine the interaction of nephron number and UUO, studies were initiated in Os/+ mutant mice, born with a 50% reduction in nephron number. Results show that congenital partial UUO impairs glomerular and proximal tubular growth, with additional nephron loss in Os/+ mice, whose growth of remaining nephrons is not restored by release of the obstruction. Collaboration with Jared Grantham’s group at the University of Kansas allowed us to study mouse models of rapidly and moderately progressive polycystic kidney disease, a genetic disorder characterized by expanding tubular cysts leading to kidney failure. This work led to the conclusion that expanding cysts obstruct adjacent nephrons, with a pathogenesis similar to that resulting from UUO.
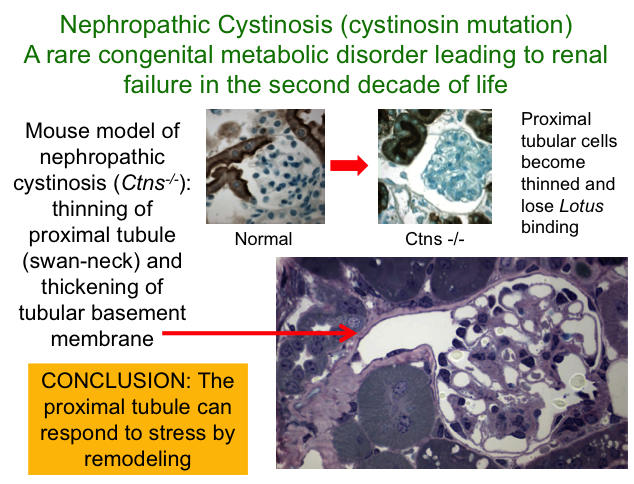 We also established collaboration with Corinne Antignac’s group (Hôpital Necker, Paris) who recently developed a mutant mouse model of nephropathic cystinosis, a rare but devastating inherited disease that causes injury to the proximal tubules, leading to kidney failure in children. Our studies of these animals revealed that in contrast to rapid cell death of proximal tubules (which results from severe UUO), these cells adapt to a genetic defect in amino acid transport by changing their shape and function. Although this temporizes the effects of oxidative stress by transferring glomerular filtrate to more distal segments, the mice die of renal failure in late adulthood. Treatment of cystinotic mice with a novel antioxidant directed at mitochondria (mostly localized in the kidney to proximal tubules) slowed progression of the proximal tubular lesions. These results suggest new potential treatments for children with kidney disease, with the goal of delaying or preventing the need for dialysis or transplantation.
We also established collaboration with Corinne Antignac’s group (Hôpital Necker, Paris) who recently developed a mutant mouse model of nephropathic cystinosis, a rare but devastating inherited disease that causes injury to the proximal tubules, leading to kidney failure in children. Our studies of these animals revealed that in contrast to rapid cell death of proximal tubules (which results from severe UUO), these cells adapt to a genetic defect in amino acid transport by changing their shape and function. Although this temporizes the effects of oxidative stress by transferring glomerular filtrate to more distal segments, the mice die of renal failure in late adulthood. Treatment of cystinotic mice with a novel antioxidant directed at mitochondria (mostly localized in the kidney to proximal tubules) slowed progression of the proximal tubular lesions. These results suggest new potential treatments for children with kidney disease, with the goal of delaying or preventing the need for dialysis or transplantation.

Chevalier Lab (left to right): Lauren Simpkins (UVa Echols Scholar, undergraduate); Carolina Galarreta, MD (Research Associate); Maria Sergio, MD (Pediatric surgeon and Fulbright Scholar from University of Palermo); Bobi Thornhill (animal surgeon); Michael Forbes, PhD (microscopist); Robert Chevalier, MD (Principal Investigator)
