Regenerative capacity of the kidney after ureteral obstruction release
About
The overall goal of this research project is to identify the cellular precursors and mechanisms underlying the regeneration of the kidney observed after ureteral obstruction release. Congenital obstructive nephropathy is the leading cause of chronic kidney disease in children, and its management is currently limited to surgical release of the obstruction. Indications for intervention as well as its timing are highly controversial, and the procedure often fails to prevent progressive loss of renal function. Understanding the cellular responses to chronic urinary tract obstruction, as well as to release of the obstruction, may reveal new therapies to slow or prevent nephron loss. The experimental approach will be to use a well-characterized model of chronic partial
(p) unilateral ureteral obstruction (UUO) in the neonatal mouse, which replicates urinary tract obstruction in the midtrimester human fetus. Surgical release of the obstruction leads to arrest of tubular injury and regeneration plus remodeling of renal parenchyma.
Our preliminary data show that during neonatal pUUO there is a major loss of not only proximal tubular cells (resulting in atubular glomeruli) but also of collecting duct cells in addition to the expansion of interstitial cells ultimately leading to fibrosis. Upon release of the obstruction, there is a striking reversal of the damage with regeneration of both proximal tubules and collecting ducts, from precursors of uncertain origin. We therefore propose to identify, isolate and characterize the precursors and mechanisms whereby those precursors repair the massive kidney damage produced by obstruction. The proposed work will also fill an important gap in our knowledge regarding potential gender differences, and identification of the therapeutic window for surgical release of the obstructed kidneys. This proposal will test the interrelated hypotheses that repair of the kidney after release of ureteral obstruction occurs by neogenesis of compartment-specific precursors and that Sox9 is required for the normal regenerative capacity of the tubular and interstitial compartments (Figure 1).
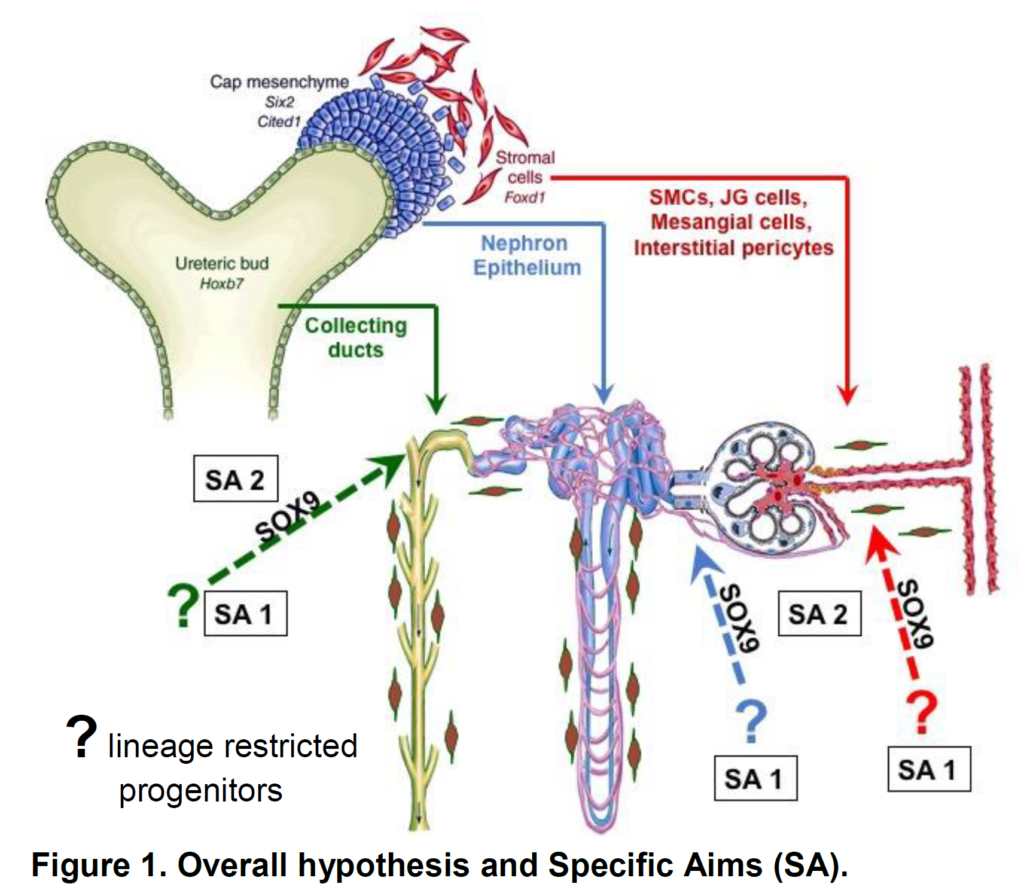
Specific Aims
Aim1 Define the regenerative capacity of the
postnatal metanephric kidney following obstructive nephropathy.
Aim1a. Determine the window of regenerative capacity of the obstructed kidney, the cell lineages
involved and whether there is any difference related to sex.
Aim1b. Identify and characterize precursors involved in kidney regeneration/repair after ureteral
obstruction release.
In this aim we will use a model of reversible pUUO in the neonatal mouse, which parallels urinary tract obstruction in the human fetus, on well characterized transgenic mice with Cre recombinase and fluorescent reporter expression restricted to tubular or stromal cells. Analysis by quantitative immunohistochemistry, Fluorescent Activated Cell Sorting and single cell profiling will allow us to identify cell types and key gene circuits that can be used as targets for kidney regeneration.
Aim2 Determine the role of Sox9 in regeneration of the tubular and interstitial compartments during
pUUO and after release.
Aim2a. Define whether Sox9 is required for the normal regenerative capacity of the tubular and
interstitial compartments.
Aim2b. Determine whether overexpression of Sox9 in tubular and interstitial compartments diminishes
the injury due to pUUO and increases the regenerative capacity after release.
In this aim we will use the same model of reversible pUUO in neonatal mice with inducible expression of Cre recombinase and concomitant fluorescent reporter expression to delete (Aim2a) and overexpress (Aim2b) the transcription factor Sox9.
The proposed experiments will solve an existing challenge and generate new and exciting information of relevance to the fields of kidney regeneration with the potential to benefit children and adults with kidney diseases.
