Epigenetic mechanisms of nephron progenitor cell renewal and fate
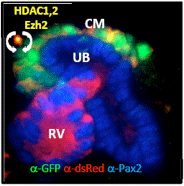
Epigenetic regulation of Nephron Progenitor Cell (NPC) homeostasis. Renewal of the cap mesenchyme (CM) depends on the concerted activities of Class I histone deacetylases (HDAC 1&2) and the polycomb histone lysine 27 methyltransferase Ezh2. Kidney section from E14.5 R26R(dsRed):Six2‐GFP.Cre mouse. Green: GFP (Six2+); Red: dsRed+ descendant of Six2+ cells. CM: cap mesenchyme; RV: renal vesicle
Although the scientific evidence linking low nephron number to progressive renal disease is well established, the basic mechanisms which determine nephron endowment are not completely understood. Six2+ cells in the cap mesenchyme are the nephron “stem-like” cells. Self-renewal and lineage-specific differentiation are essential properties of these multipotent progenitor cells. Failure of either of these processes disrupts nephrogenesis and thus predisposes to chronic kidney disease and hypertension. This grant application examines how epigenetic chromatin-based mechanisms control nephron progenitor cell maintenance and differentiation. Transcriptionally silent genes can be marked by histone modifications and regulatory proteins that indicate the genes’ potential to be activated. Such bivalent marks have been identified in pluripotent cells, but it is unknown how such marks occur in descendant cells that have restricted cell fate choices. Furthermore, it is not known whether enzymes that establish chromatin states can control embryonic fate choices. Genome-wide chromatin maps included in the proposal strongly suggest that nephron progenitor cells are epigenetically” poised”. Indeed, targeted deletion of the histone lysine 27 methyltransferase, Ezh2, from the cap mesenchyme disrupts self-renewal of progenitors and drives precocious differentiation. Inactivation of histone deacetylases, HDAC1&2, which act as partners in the Ezh2/PRC2 complex, also disrupts nephron progenitor homeostasis. The overall hypothesis to be tested in this proposal is that the histone methylation and acetylation machineries are essential for nephron progenitor cell renewal and fate. In this proposal we will determine that chromatin bivalency, which is partly mediated by Ezh2, is a requisite to maintain silent differentiation genes in a poised state. We will also investigate the functional cross-talk between histone deacetylases and methyltransferases in nephron progenitor cell maintenance and differentiation. Finally, our studies will delineate the transcriptional regulatory networks downstream of Ezh2 and HDAC1/2 which control nephron progenitor cell multipotency.
Approximately 40% of chronic kidney disease cases in children are caused by congenital anomalies of the kidney and urinary tract. This project will provide insight into the regulation of kidney progenitor cell differentiation. This will have significant impact on diseases that affect progenitor cell maintenance such as renal hypoplasia and Wilms tumor, as well as on therapies that depend on stem cell regeneration.
