RBPJ and cell fate in the kidney vasculature
Specific Aims
The unique spatial arrangement of the kidney arterioles with each nephron is crucial for the regulation of renal blood flow, glomerular filtration rate and other specialized kidney functions that maintain homeostasis. Thus, the proper and timely assembly of the arterioles with their respective nephrons is a crucial morphogenetic event leading to the formation of a functioning kidney necessary for independent extra uterine life. The mechanisms that govern the development of the kidney vasculature are poorly understood.
We have shown that Foxd1+ cells and their descendants, Renin Precursor Cells (RPCs) are the earliest metanephric progenitors for all the mural cells of the kidney arterioles including JG cells, pericytes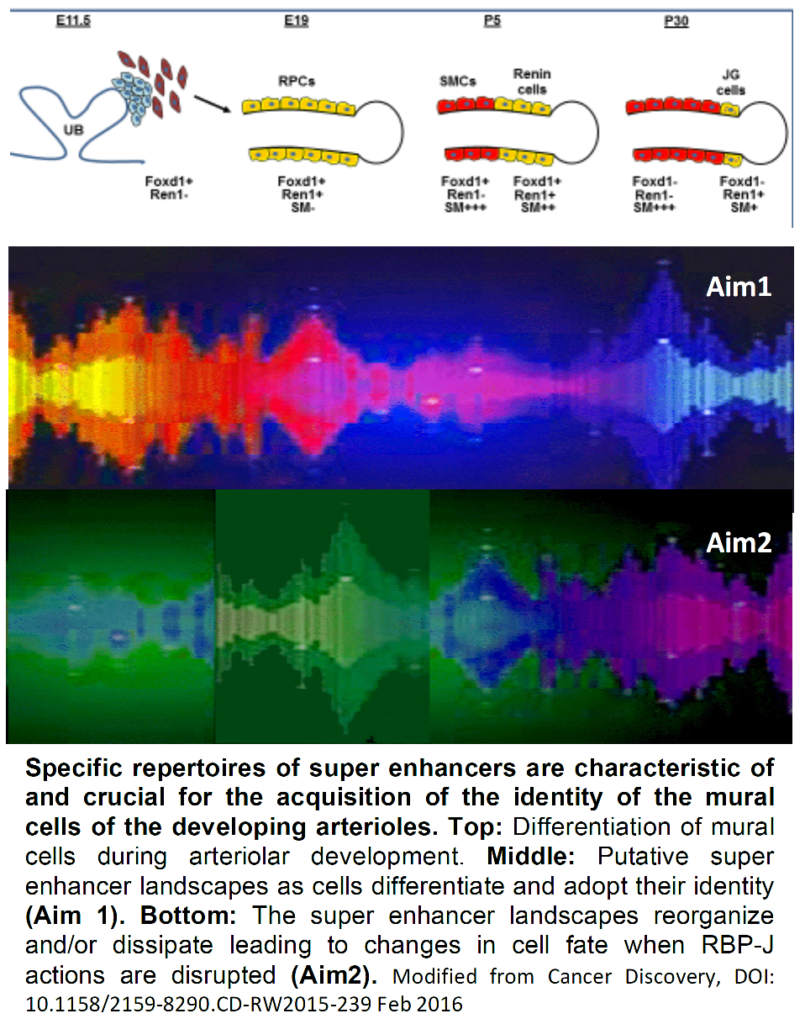 , arteriolar smooth muscle cells (SMCs) and mesangial cells. Recently, we found that RPCs harbor Super- Enhancers (SEs): clusters of large genomic regulatory regions that drive expression of genes that define cell identity. SEs are formed by high density of transcription factor (TF) binding to DNA, deposition of co- activators (Mediator, p300), distinctive histone marks such as H3K27ac and open chromatin configuration. Further, we showed that RBP-J, the final transcriptional effector for all Notch receptors, governs a network of genes involved in the acquisition and maintenance of the endocrine-contractile phenotype of the renin precursor cells. Cells from mutant mice lose their characteristic myo-epithelioid granulated phenotype and are unable to make granules, synthesize renin or smooth muscle proteins. Remarkably, the RBP-J -/- mutant cells switch phenotype and express genes usually encountered in hematopoietic cells indicating that an additional key function of RBP-J is to repress the ectopic expression of genes from undesirable lineages. We hypothesize that SEs are established early during differentiation of the mural cells of the kidney arterioles and that RBP-J is crucial in the organization of the chromatin landscape genome-wide and in the operation of the renin SE in particular.
, arteriolar smooth muscle cells (SMCs) and mesangial cells. Recently, we found that RPCs harbor Super- Enhancers (SEs): clusters of large genomic regulatory regions that drive expression of genes that define cell identity. SEs are formed by high density of transcription factor (TF) binding to DNA, deposition of co- activators (Mediator, p300), distinctive histone marks such as H3K27ac and open chromatin configuration. Further, we showed that RBP-J, the final transcriptional effector for all Notch receptors, governs a network of genes involved in the acquisition and maintenance of the endocrine-contractile phenotype of the renin precursor cells. Cells from mutant mice lose their characteristic myo-epithelioid granulated phenotype and are unable to make granules, synthesize renin or smooth muscle proteins. Remarkably, the RBP-J -/- mutant cells switch phenotype and express genes usually encountered in hematopoietic cells indicating that an additional key function of RBP-J is to repress the ectopic expression of genes from undesirable lineages. We hypothesize that SEs are established early during differentiation of the mural cells of the kidney arterioles and that RBP-J is crucial in the organization of the chromatin landscape genome-wide and in the operation of the renin SE in particular.
Aim 1 will test the hypothesis that during morphogenesis of the renal arterial tree, cell-specific SEs determine the identity and fate of the mural cells of the renal arterial tree.
Aim 2 will test the hypothesis that RBP-J is crucial for the acquisition and maintenance of the chromatin landscape and the operation of the SEs that control the fate of the mural cells of the kidney arterioles.
By providing fundamental knowledge regarding the mechanisms whereby progenitor cells contribute to the endowment of renal vascular cells and how those cells differentiate and assemble to form the renal arterioles, a frontier basically unexplored, this proposal has the potential to benefit children and adults with congenital and acquired kidney diseases, vascular diseases and hypertension.
