Jennifer C. Burnsed, MD, MS

Hypoxic-ischemic encephalopathy (HIE) is a condition that affects 1.5/1000 newborns annually in developed nations and may lead to death, seizures, cerebral palsy, and behavioral and cognitive defects. These cognitive and behavioral deficits are difficult to predict in neonatal animals and humans based on available imaging and exam. This suggests there are microstructural or functional changes in neuronal circuitry and activation that are not detected during standard imaging (MRI) and neurologic examination. These changes may later manifest as deficits in memory, learning, and behavior. Disruption of hippocampal circuitry associated with HIE may shed light on these changes. Our goal is to provide new fundamental knowledge regarding mechanisms of behavioral and cognitive deficits following HIE and improve outcomes for infants with HIE.
Cognitive and behavioral outcomes following neonatal hypoxic-ischemic encephalopathy (HIE) are difficult to predict during the neonatal period. Deficits in working and spatial memory exist in young adult mice that were exposed to neonatal hypoxia-ischemia. Examining a well-established neonatal mouse model of hypoxic-ischemic brain injury using novel methods, we have found that a subset of young adult mice exhibit interictal spikes and chronic abnormal neuronal activation in the hippocampal-parahippocampal circuit. We propose that memory function is disrupted by interictal spikes and chronically abnormal neuronal activity in the hippocampal-parahippocampal circuit related to neonatal HIE. We characterize electrographic abnormalities and neuronal activity in the hippocampal circuitry in young adult mice following neonatal hypoxia-ischemia using neuronal activation mapping in lipid cleared transgenic mouse brains and high quality electrographic recordings. We are investigating the link between electrographic abnormalities, abnormal neuronal activity in hippocampal circuitry and behavioral deficits in this model.
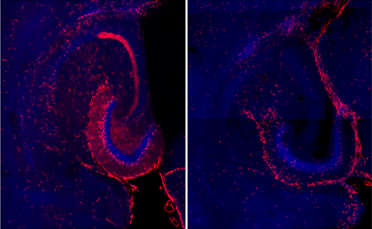
- Active neurons (red) in hippocampus following neonatal hypoxia ischemia (left) vs. control (right).
- Intact neonatal mouse brain rendered transparent using tissue clarifying process.