Santina Zanelli, MD
 Our research focus is to understand the molecular mechanisms regulating seizure generation in the developing brain. Neonatal seizures are a common problem and remain a frustrating clinical challenge with little agreement on diagnosis or treatment goals. The problem is further compounded by the disappointing efficacy of currently available anti-seizure medications. The lack of progress in improving treatment algorithms for these patients is partly due to an incomplete understanding of the mechanisms underlying seizure generation in an immature brain, particularly in the acute phase. Previous studies have focused on the excitation/inhibition imbalance as a primary cause for increased seizure susceptibility in neonates; however, the large amount of knowledge gained in this area has failed to lead to more effective or safer therapies for these patients. The goal of our research is to advance our understanding of the pathophysiology of seizures in the neonatal period, focusing on seizures caused by a hypoxic or hypoxic-ischemic insult (the most common cause of seizures in human neonates).
Our research focus is to understand the molecular mechanisms regulating seizure generation in the developing brain. Neonatal seizures are a common problem and remain a frustrating clinical challenge with little agreement on diagnosis or treatment goals. The problem is further compounded by the disappointing efficacy of currently available anti-seizure medications. The lack of progress in improving treatment algorithms for these patients is partly due to an incomplete understanding of the mechanisms underlying seizure generation in an immature brain, particularly in the acute phase. Previous studies have focused on the excitation/inhibition imbalance as a primary cause for increased seizure susceptibility in neonates; however, the large amount of knowledge gained in this area has failed to lead to more effective or safer therapies for these patients. The goal of our research is to advance our understanding of the pathophysiology of seizures in the neonatal period, focusing on seizures caused by a hypoxic or hypoxic-ischemic insult (the most common cause of seizures in human neonates).
To address some of these questions, we developed a novel model of hypoxic seizures in neonatal mice. We were able to record high quality EEGs in postnatal mice starting at postnatal day 2. Using this model, we demonstrated that exposure to acute severe hypoxia during the early postnatal period leads to seizures both during hypoxia and during early reoxygenation and that response to hypoxia varies with postnatal age and maturation. These seizures were more commonly observed in animals P3-P10. Of note, exposure to this degree and duration of hypoxia was uniformly fatal in older animals. Importantly, early reoxygenation seizures were common and did not have clinical correlate in the neonatal period emphasizing the crucial role of continuous EEG monitoring when studying neonatal seizures.
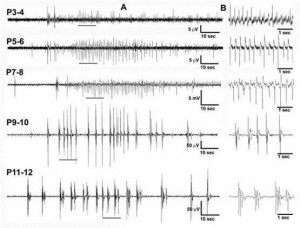 Fig. 1 Effects of postnatal age on hypoxic-seizures in the neonatal mouse
Fig. 1 Effects of postnatal age on hypoxic-seizures in the neonatal mouse
We are also investigating the effects of hypoxia on synaptic transmission in the hippocampus using electrophysiology techniques. We found that in vitro ischemia alters CA1 pyramidal neurons membrane intrinsic properties leading to hyperexcitability but also lead to increased excitatory synaptic transmission in the neonatal mouse. Further, both pre-synaptic and post-synaptic mechanisms appear to play an important role in this observation. While investigating these mechanisms, we discovered that kainate receptor activation modulates, at least in part, some of this response. Kainate receptors have recently been the subject of increased scrutiny and there is growing evidence that they play an important role in the pathophysiology of seizures and epilepsy. They are abundantly expressed in the brains of neonatal animals and have diverse functions important to the regulation of neuronal network activity. However, gaps in knowledge remain in the understanding of their role in modulating response to hypoxia and seizure generation in the neonatal period. Our current work focuses on characterizing the role of these receptors in modulating susceptibility to hypoxic seizures during the neonatal period.
Finally, we are also interested in understanding the effects of early-life seizures on neuronal activation in the mouse hippocampus. Spontaneous, synchronized network activity has been shown to occur in the developing nervous system and is essential for the generation of functional circuits. The effects of hypoxia on such circuits are not known. To answer this question, we are also studying the activation patterns and spatial responses of hippocampal neurons during in vitro asphyxia using the genetically encoded calcium indicators SynGCaMP6. We also can temporally assess cFos activation following hypoxia in cFos-GFP transgenic mice using advanced microscopy techniques in clarified brains. Ultimately, the goal of our research is to develop safer, more effective, mechanism-based therapies for neonates with early-life seizures.
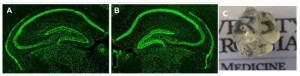
Effects of hypoxia on cFos expression in the neonatal mouse hippocampus.
Panel A: Control P7 mouse.
Panel B: 24h post-hypoxia mouse. We found a 61 ± 24%; 19 ± 7% and 62 ± 50% increase
in fluorescence in CA1, CA3 and DG, respectively. Images analyzed in Image J, Corrected total area fluorescence shown.
Panel C: Clarified whole brain in P7 mouse.
Contact Information:
Phone: 434-924-2521
Email: sc5d@virginia.edu