R. Ariel Gomez, MD
 The goal of our research program is to define the mechanisms that govern the identity and plasticity of renin-expressing cells. The answer to this question had been rather elusive because: 1) the cells are few in number (0.01 % of the total kidney cell mass), 2) it had been impossible to isolate them to purity, 3) they switch phenotype on and off, 4) when in culture they stop making renin after 48 h, and 5) there were no other markers that could identify these cells independently of renin. To circumvent those major problems, we developed a variety of genetically engineered mice models and in vitro dually labeled cell culture systems that allowed us to identify, trace the lineage and fate of renin cells during normal development and in response to physiological and pathological challenges. Because the cells are endogenously labeled with vital markers (CFP, GFP, and YFP) we could traced them in vivo, isolate them by FACS and study them with precision. Briefly, we demonstrated that: 1) renin cells originate from Foxd1+ mesenchyme cells within the kidney 2) renin cells are themselves precursors for arteriolar smooth muscle cells, pericytes, mesangial cells, and a subset of tubular cells 3) the cAMP pathway is a major determinant of renin cell identity, an effect that is mediated by chromatin remodeling- namely histone 4 acetylation- at the cAMP element of the renin gene 4) histone acetyl transferases (CBP and p300) are crucial in the maintenance of the renin phenotype 5) renin cells are directly involved in the branching and elongation of the kidney vasculature, 6) RBP-J, the final common effector of the Notch signaling pathway, is required to maintain the number of renin-expressing cells, and it is crucial in the plasticity of vascular SMCs and mesangial cells to regain the renin phenotype in response to a threat to homeostasis. 7) Expression profile analysis revealed that renin cells express a unique set of genes characteristic of the renin phenotype, which is vastly different from other cell types in the kidney.
The goal of our research program is to define the mechanisms that govern the identity and plasticity of renin-expressing cells. The answer to this question had been rather elusive because: 1) the cells are few in number (0.01 % of the total kidney cell mass), 2) it had been impossible to isolate them to purity, 3) they switch phenotype on and off, 4) when in culture they stop making renin after 48 h, and 5) there were no other markers that could identify these cells independently of renin. To circumvent those major problems, we developed a variety of genetically engineered mice models and in vitro dually labeled cell culture systems that allowed us to identify, trace the lineage and fate of renin cells during normal development and in response to physiological and pathological challenges. Because the cells are endogenously labeled with vital markers (CFP, GFP, and YFP) we could traced them in vivo, isolate them by FACS and study them with precision. Briefly, we demonstrated that: 1) renin cells originate from Foxd1+ mesenchyme cells within the kidney 2) renin cells are themselves precursors for arteriolar smooth muscle cells, pericytes, mesangial cells, and a subset of tubular cells 3) the cAMP pathway is a major determinant of renin cell identity, an effect that is mediated by chromatin remodeling- namely histone 4 acetylation- at the cAMP element of the renin gene 4) histone acetyl transferases (CBP and p300) are crucial in the maintenance of the renin phenotype 5) renin cells are directly involved in the branching and elongation of the kidney vasculature, 6) RBP-J, the final common effector of the Notch signaling pathway, is required to maintain the number of renin-expressing cells, and it is crucial in the plasticity of vascular SMCs and mesangial cells to regain the renin phenotype in response to a threat to homeostasis. 7) Expression profile analysis revealed that renin cells express a unique set of genes characteristic of the renin phenotype, which is vastly different from other cell types in the kidney.
Current work in the laboratory is focused on the understanding the epigenetic events and gene-gene interactions that determine the renin cell phenotype.
In collaboration with Dr. Sequeira-Lopez and Dr. Belyea we are defining the lineage relationships, differentiation and function of renin cells and their precursors in extra renal hematopoietic tissues. In this regard, we have recently discovered a renin precursor in the bone marrow, a B lymphocyte that expresses renin and constitutes the cell of origin of a highly penetrant and aggressive type of B cell leukemia.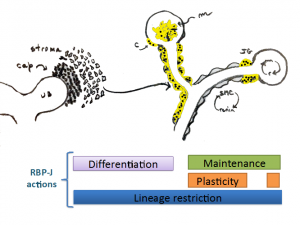
During early vascular development RPB-J regulates differentiation and morphogenesis of the kidney vasculature by controlling the fate of mesangial cells and the mural cells of the kidney vasculature. Later in life the role of RBP-J/Notch ,may be to maintain the differentiate state and the plasticity of SMCs to regain the renin phenotype. All the while, the most important function preventing the expression of unwanted/undesirable genes, lineage restriction.
Contact Information:
Phone: 434-243-0867
Email: rg@virginia.edu
