Brian Belyea, MD
Role of Renin Progenitors in Hematopoiesis
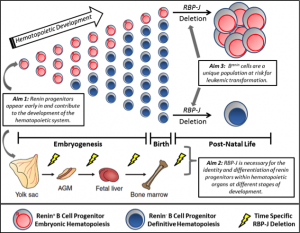
We have recently made the exciting observation that renin-expressing progenitors are present in the bone marrow, and that these cells serve as the cell of origin for B cell leukemia. While pursuing the mechanisms that regulate the identity of renin cells, we conditionally deleted the Notch mediator RBP-J in renin-expressing cells. Upon deletion of RBP-J, these cells experienced developmental arrest, cell cycle progression, and ultimately development of a highly penetrant and fulminant B cell leukemia in mice.
Renin cells have traditionally been associated with the kidney, where they function to regulate blood pressure and fluid-electrolyte homeostasis. However, lineage studies in the Gomez – Sequeira Lopez laboratories showed that renin cells are progenitors for multiple cell types in the kidney and extrarenal organs. Although the function of renin cells in the kidney is understood, the role of renin cell progenitors in the development of the bone marrow and other hematopoietic organs is not clear. We recently found that renin is expressed in the bone marrow and spleen during embryonic and post-natal life in mice. Most of these renin-expressing hematopoietic cells have cell surface markers, gene expression, and growth characteristics of B lymphocyte progenitors. Further, these cells represent a subset of total B lymphocytes, are numerous at birth, and diminish with age, suggesting renin expression may be prominent during embryonic hematopoiesis.
However, it is unknown when renin cells first appear during hematopoietic development, when RBP-J becomes critical to normal hematopoiesis, and if renin progenitors are unique in their susceptibility to malignant transformation. Given the potential medical relevance of these findings, we are pursuing experiments to fully characterize this novel progenitor cell during hematopoietic ontogeny and its contribution to the establishment and differentiation of hematopoietic organs. The overall hypothesis we are testing is that renin-expressing hematopoietic cells are a primitive component of the hematopoietic system with differential transformative capacity, and that RBP-J is necessary for the appropriate fate of these cells.
Defining a Tumor Suppressor Role of RBP-J in B Cell Progenitors
Precursor B-cell acute lymphoblastic leukemia (B-ALL) is the most common childhood malignancy, affecting ~3,000 children each year in the United States. It is critical to understand the underlying cytogenetic and molecular events driving leukemogenesis as they serve as independent prognostic determinants, are used to refine risk stratification and assign treatment intensification, and serve as markers of residual disease. Many of the currently known genetic aberrations in B-ALL affect genes involved in B cell development. In fact, genes regulating normal B cell development are altered in over 40% of precursor B cell acute leukemia cases. However, up to 25% of B-ALL cases have no identifiable genetic alteration. It is possible that genes involved in B cell development which are not yet recognized may be altered or that epigenetic events may be playing a primary role in these remaining cases.
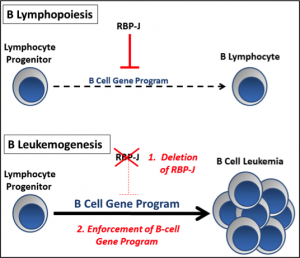
The Notch signaling pathway is an evolutionarily conserved pathway which has been shown to regulate multiple steps during hematopoiesis, including fate choices of progenitors. While there are 5 Notch ligands and 4 Notch receptors in mammals, the transcription factor RBP-J functions immediately downstream and mediates the transcriptional activation of all 4 Notch receptors. During lymphopoiesis, Notch/RBP-J signaling functions to promote T cell development over B cell fates in lymphocyte progenitors by repressing genes important for B cell development. Aberrant regulation of Notch signaling can lead to pathologic cell transformation, and an association between activating Notch mutations and T-cell leukemia has previously been noted in humans. In contrast, activation of the Notch pathway appears to cause growth arrest in a wide range of B cell malignancies. However, a causal or initiating role of Notch deficiency in B cell malignancies has not been previously described.
Our lab has recently developed a highly penetrant model of precursor B cell leukemia by silencing the Notch mediator RBP-J in a subset of B-cell progenitors which express renin. When RBP-J is silenced, there is enrichment of a B cell gene program leading to increased transcription of B-lineage associated genes. In addition, these cells experience developmental arrest and a proliferative advantage ultimately leading to precursor B cell leukemia. Taking advantage of this mouse model and using other techniques performed routinely in our lab such as tissue-specific recombination, lineage tracing, and flow cytometry, we are further exploring the tumor suppressor role of Notch/RBP-J during normal and neoplastic B lymphopoiesis. The overall theme being tested is that RBP-J functions as a tumor suppressor in B lymphocyte progenitors and that silencing of RBP-J enriches and enforces a precursor B cell genetic program.
Jagged1 and Kidney Vascular Development
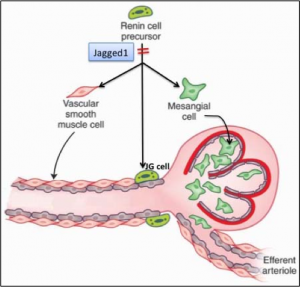
Renal vascular development is dependent on the participation of renin precursor cells, which localize to areas of new vessel formation and differentiate into smooth muscle cells (SMCs), mesangial and JG cells. However, the mechanisms that enforce this particular fate of renin precursor cells remain unclear. The Notch signaling pathway is involved in the regulation of vascular development. Dr. Gomez’s laboratory has previously shown that during embryogenesis, renin cells express a significant number of angiogenic factors including Notch receptors and their ligands. Notable among these factors is Jagged 1, which is highly expressed in renin cell precursors suggesting that it may specify renin cells to a SMC cell, JG cell and/or a mesangial cell fate. We therefore hypothesize that Jagged1 is necessary for renin precursors to differentiate into the various mural cells of the renal arterioles and that Jagged1 plays a critical role in renal vascular development.
We are currently studying the expression pattern of Jagged 1 at different times during development and post-natal life, and investigating whether conditional Jagged1 deletion in the renin lineage cells alters the differentiation of renin cell precursors to arterial SMCs, mesangial cells and JG cells and overall renal vascular development. We predict that Jagged1 is required for activation of the Notch signaling pathway in the renin cell lineage and that continuous expression is necessary for maintaining cell fate and plasticity. Lack of Jagged 1 may lead to downregulation of the Notch signaling pathway in renin cells and ultimately loss of their ability to differentiate into multiple cell fates including vascular SMCs, mesangial cells, and JG cells. The proposed experiments should yield significant and relevant information to our understanding of renal vascular development.
Cells of the Renin Lineage in the Peritoneal Cavity
Renin expressing cells are crucial in the control of blood pressure and fluid-electrolyte homeostasis. The expression of renin is likely regulated by direct interaction between renin cells and other, adjacent cell types. The Notch signaling pathway is an evolutionary conserved pathway which is vital for cell-cell communication during fundamental developmental processes. In addition, members of the Notch signaling pathway are expressed in renin expressing cells of the kidney. Therefore, our lab hypothesized that the Notch signaling pathway, conveyed by cell-cell signaling, may be crucial in maintaining the renin cell phenotype. To that end, our lab previously used a conditional knockout approach to delete the final effector of Notch signaling, RBP-J, specifically within cells of the renin lineage. Conditional deletion of RBP-J in renin cells resulted in a severe reduction in the number of renin-positive cells in the kidney. This work showed that RBP-J is required to maintain renin expression within JG cells.
Unexpectedly, as these mice aged beyond ~6 months, they developed signs and symptoms of leukemia. Animals developed striking splenomegaly, leukocytosis, and hypercellular bone marrow consisting of a homogeneous blast population. We then investigated which cells within the hematopoietic system are capable of producing renin and serve as the origin for this leukemia model. Using lineage tracing strategies, we demonstrated renin-lineage cells within the spleen and bone marrow. Flow cytometry, gene array, and culture studies demonstrated that renin cells are precursor B lymphocytes. Of interest, the resulting B-cell leukemia phenotype is characterized by dim B220 expression. We therefore questioned if renin expressing cells (which serve as the cell of origin for this model of leukemia) may be related to a specialized subset of B-lymphocytes with dim B220 expression – B-1 lymphocytes. B-1 lymphocytes differ functionally and phenotypically from conventional B-2 B cells.
We generated Ren1dcre/+;mT/mG mice which after Cre-mediated recombination express GFP in renin precursors and their descendants and then performed lineage tracing and transplantation studies. We found that cells from the renin lineage are enriched in the peritoneal fluid when compared to bone marrow, blood and spleen. In addition, we found evidence of renin expression within peritoneal cells (sqRT-PCR and transgenic reporter studies). Whereas the proportion of cells from the renin lineage in bone marrow, blood, and spleen decreases with age, renin lineage B-1 cells within the peritoneal fluid persist into adulthood. Further, while renin-expressing cells in the bone marrow are pro-B cells (B-2 cells), cells from the renin lineage within the peritoneal fluid exhibit increased expression of CD11b and CD5, and overall have an immunophenotype consistent with B-1 lymphocytes. Finally, we found that renin lineage cells from the bone marrow of donor mice can repopulate the B-1b cell peritoneal compartment in irradiated host mice. In fact, renin lineage cells were more efficient in giving rise to B-1b cells compared to non-renin lineage cells suggesting that renin expression marks an early subset of cells that become B-1 cells. This preliminary work suggests an association between renin, the key regulatory enzyme of the renin angiotensin cascade and the innate immune system, two major systems in the control of homeostasis. Renin lineage B-1 cells within the peritoneal cavity may be involved in defense of infection and/or be involved in the regulation of blood pressure and electrolyte changes. Further studies are underway to more fully characterize this cell population.
Contact Information:
Phone: 434-924-8499
Email: bcb9e@virginia.edu