Omar Guessoum1, Maria Luisa S. Sequeira-Lopez2, Ariel Gomez1,2
1 Department of Biology, University of Virginia, Charlottesville, VA 2 Department of Pediatrics, Division of Nephrology, University of Virginia School of Medicine.
Mentors: Ariel Gomez & Maria Luisa Sequeira-Lopez, Department of Pediatrics, Division of Nephrology.
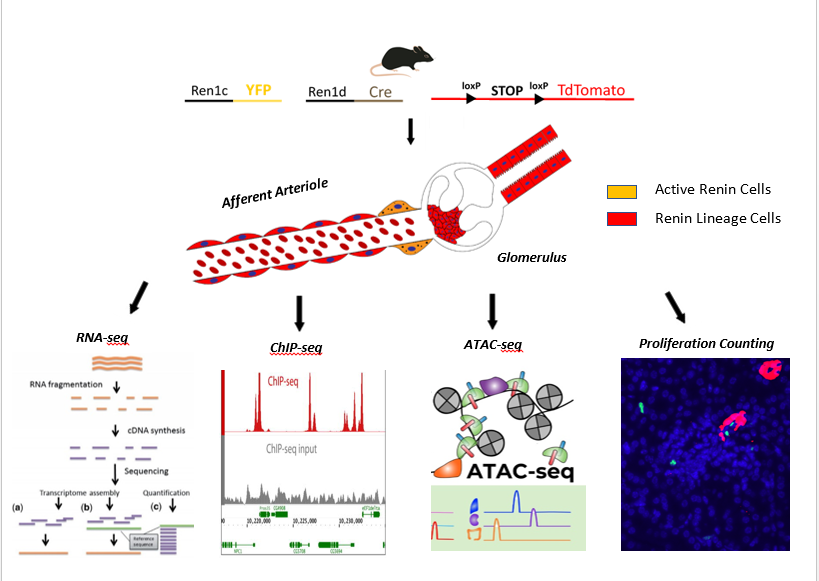
Background: The Renin-Angiotensin-System (RAS) is essential to maintain blood pressure and fluid electrolyte homeostasis. The rate limiting step of the system is the activity of the hormone-enzyme renin which is released from juxtaglomerular (JG) cells of the kidney. In response to reduction in blood pressure, the number of renin cells in the kidney increase leading to a rise in circulating levels of renin. One way renin cell number can be increased is by phenotypic switching of neighboring vascular smooth muscle cells into renin cells. Early in development, renin expression is widespread and these progenitors give rise to numerous cell types including vascular smooth muscle, proximal tubules and collecting ducts. These cells, smooth muscle in particular, have a molecular memory of the renin cell program which they can activate during stress. The nature of this molecular memory is unknown and could involve epigenetic regulation of genes central to the identity of the renin cell. Alternatively, increased renin production could be accomplished by proliferation of pre-existing JG cells.
Objectives: 1) To test the hypothesis that renin lineage cells have permissive chromatin landscapes at genes central to the renin cell identity. 2) To test whether proliferation of renin cells contributes to increased renin production during stress.
Design and Methods: 1) Using the Cre-recombinase system, we labelled cells of the renin lineage with TdTomato and active renin cells with a transgenic YFP reporting renin expression allowing their isolation via FACS. We then performed ChIP-seq for H3K4me3 (an activating epigenetic mark), ATAC-seq to find open chromatin regions and RNA-seq to study the transcriptome 2) We performed co-immunofluorescence for renin & proliferation markers and quantified the # of co-localizations. We also performed RNA-seq on renin cells isolated using the YFP transgene to determine whether there was an increase in expression of proliferation markers in response to treatment with drugs that lower blood pressure.
Results: 1) Using immunofluorescence, we found few dividing renin cells under basal physiological conditions and this was not significantly increased during homeostatic stress. 2) RNA-seq of recruited renin cells showed negligible expression of proliferation markers indicating that JG cells do not proliferate to restore homeostasis 3) Using ATAC-seq and ChIP-seq on renin lineage cells, we found no signal at the renin gene or genes associated with smooth muscle, suggesting these cells are predominantly non-muscle 4) RNA-seq on renin lineage cells revealed enrichment of tubular and collecting duct genes which likely diluted the smooth muscle cells.
Conclusions: Our studies indicate that the contribution of proliferation to the increase in renin cells during physiological stress is negligible. Furthermore, our epigenetic studies revealed that cells of the renin lineage have a closed renin regulatory region and lack activating marks. We propose that this was the result of the prevalence of tubular and collecting duct cells in the renin lineage and future studies will address this issue by studying only those smooth muscle cells that can adopt the renin cell identity.
Summary
- Objectives: 1) To test the hypothesis that renin lineage cells have permissive chromatin landscapes at genes central to the renin cell identity. 2) To test whether proliferation of renin cells contributes to increased renin production during stress.
- Conclusions: Our studies show that proliferation makes a negligible contribution to recruitment. Furthermore, our epigenetic studies revealed that cells of the renin lineage do not have activating/poised marks which can be attributed to tubular and collecting duct cells diluting the smooth muscle cells which can be recruited.
- Implications for Children: Understanding the epigenetic regulation involved in the plastic ability of renin cells to switch between renin and smooth muscle cell fate has the potential to improve the health of children and adults afflicted by renal disease and hypertension.
*Funded by the American Heart Association Pre-doctoral Fellowship 18PRE34020090 and by National Institutes of Health Grants DK-096373 and HL-096735 to R.A.G, and DK-091330 and DK-96373 to M.L.S.S.L